

The Need
High performing PCR tests are available for large, batch analyzers but have limited practical use for clinicians due to a lag in reportable results, making appropriate same day treatment a challenge.3
Challenges remain:
- Detection and differentiation of organisms associated with bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), and trichomoniasis (TV), which all present with similar symptoms
- Mixed and co-infections of BV, VVC, and TV are common, further complicating diagnosis and the guidance of appropriate treatment4,5
- Testing options that offer accurate and objective test results to drive timely and appropriate treatment for improved patient management
2 Hillier SL, et al. Diagnosis and treatment of vaginal discharge syndromes in community practice settings. Clin Infect Dis. 2021 May 04; 72(9) 1538-1543
3 Gaydos CA, et al. Use of a Rapid Diagnostic for Chlamydia trachomatis and Neisseria gonorrhoeae for Women in the Emergency Department Can Improve Clinical Management: Report of a Randomized Clinical Trial. Ann Emerg Med. 2019 Jul; 74(1) 36-44
4 Gaydos CA, et al. Clinical Validation of a Test for the Diagnosis of Vaginitis. Obstetrics & Gynecology: July 2017 - Volume 130 - Issue 1 - p 181-189 doi: 10.1097/ AOG.0000000000002090
5 Schwebke J, et al. Clinical Validation of the Aptima Bacterial Vaginosis and Aptima Candida/Trichomonas Vaginitis Assays: Results from a Prospective Multicenter Clinical Study. J Clin Microbiol 58:e01643-19.
The Solution
On-demand molecular testing — an ideal solution:
- Actionable diagnostic aid for bacterial vaginosis, vulvovaginal candidiasis, and trichomoniasis within 60 minutes, including potential co-infections
- On-demand results for 4 separate callouts, bacterial vaginosis, Candida group,* C. glabrata/C. krusei, and T. vaginalis from a single sample collection
- Implementing easy-to-use testing with GeneXpert technology
- Approved for women 14 years old and over
The Impact
- The Xpert Xpress MVP demonstrates high sensitivity and specificity in detecting the most common causes of vaginitis from clinician and patient-collected samples in both laboratory-based and point-of-care settings.6
- Xpert Xpress MVP is a flexible, easy to use, on-demand test designed with optimized workflow efficiencies for the diagnosis of vaginitis-like symptoms.
- Accurate differential diagnosis with Xpert Xpress MVP informs appropriate treatment on time, first time, potentially improving clinical outcomes.
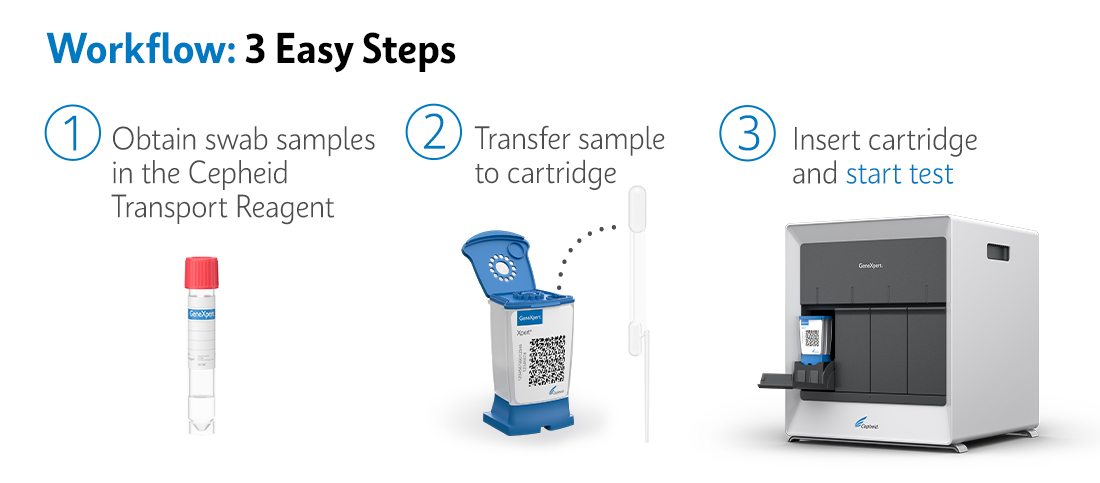
Workflow: 3 Easy Steps using the GeneXpert® System
* Detected organisms not reported individually.
^ Target includes C. albicans, C. tropicalis, C. parapsilosis, and C. dubliniensis
# Contrived specimens were prepared using individual negative clinical vaginal swab specimens.
Refer to most current Instructions for Use 301-8994 and 302-6886 (for GeneXpert Dx and Infinity Systems) for complete details
US-IVD. In Vitro Diagnostic Medical Device.
Product Resources
Product Information
Package Inserts & MSDS
ADF Files
Frequently Asked Questions
Performed on the GeneXpert® system, Xpert Xpress MVP is an automated qualitative in vitro diagnostic test for the detection of DNA targets from anaerobic bacteria associated with bacterial vaginosis (BV), Candida species associated with vulvovaginal candidiasis (VVC), and Trichomonas vaginalis (TV). (1)
Xpert Xpress MVP test uses clinician-collected and self-collected vaginal swabs (collected in a clinical setting) from patients who are symptomatic for vaginitis/vaginosis. (1)
Xpert Xpress MVP test utilizes real-time polymerase chain reaction (PCR) for the amplification of specific DNA targets and utilizes fluorogenic target-specific hybridization probes to detect and differentiate DNA from anaerobic organisms associated with bacterial vaginosis (BV), Candida species associated with vulvovaginal candidiasis (VVC) and Trichomonas vaginalis (TV). (1) A proprietary algorithm is used to determine BV status, based on the presence or absence of three anaerobic bacteria associated with BV - Atopobium spp. (Atopobium vaginae, Atopobium novel species CCUG 55226), Bacterial Vaginosis-Associated Bacterium 2 (BVAB2) and Megasphaera-1.
The Xpert Xpress MVP test is intended to aid in the diagnosis of vaginal infections in women with a clinical presentation consistent with BV, VVC, or TV. (1)
Yes, it is common. According to the literature, 17.9% of women with vaginal discharge symptoms have mixed infections, with the most common mixed infections being BV and VVC (11.2%). (2)
Yes, both Xpert Xpress MVP and Xpert CT/NG use SWAB/G-50-US.
References:
- Cepheid. Xpert Xpress MVP Package Insert. Accessed Dec. 18, 2023. https://www.cepheid.com/en-USntent/dam/www-cepheid-com/documents/package-insert-files/Xpert%20Xpress%20MVP%20US-IVD%20ENGLISH%20Instructions%20for%20Use%20301-8994%2c%20Rev%20E.pdf
- Lillis RA, Parker RL, Ackerman R, et al. Clinical Evaluation of a New Molecular Test for the Detection of Organisms Causing Vaginitis and Vaginosis. J Clin Microbiol. 2023;61(3):e0174822. doi:10.1128/jcm.01748-22.






