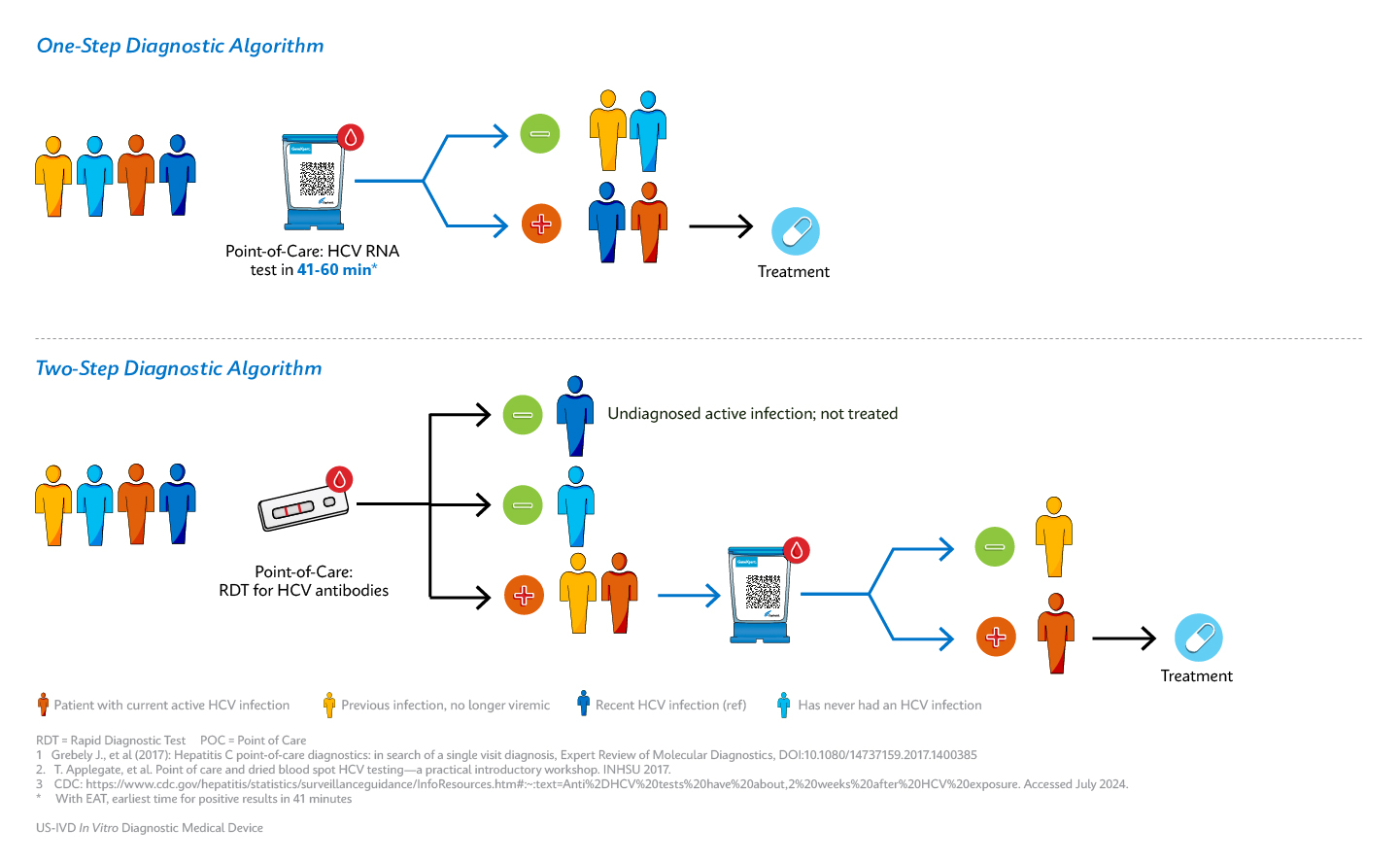
The Viral Hepatitis National Strategic Plan for the United States calls for greater than 80% of people with hepatitis C to achieve viral clearance by 20301. Currently, the diagnostic pathway for detection of hepatitis C faces many barriers, including complicated multi-step diagnostic algorithms based on antibody screening and RNA confirmation2. These algorithms require centralized testing technologies that may result in treatment delays and discourage at-risk populations from accessing testing and receiving care.


Transforming Patient Care
With Cepheid’s Xpert® HCV, patients can undergo testing, receive a diagnosis, and seamlessly connect to treatment and care in a single visit. This efficient process has the potential to save time and can ensure more patients stay in care, leading to more people cured of hepatitis C.
“This simple to use test detects the full range of relevant HCV genotypes in about an hour and can be performed on a small volume of blood collected via fingerstick.”
David H. Persing, M.D., Ph.D.
Cepheid's Chief Medical and Technology Officer
Ease of Use
1
Collect 250-500uL fingerstick whole blood in BD Microtainer®^
2
Transfer 100uL of the sample into the cartridge using the pipette provided
3
Insert cartridge and start test
IVD. In Vitro Diagnostic Medical Device. May not be available in all countries.
*K2 EDTA Microtainer (BD part number: 365974) not provided in the kit.

Cepheid’s CLIA waived Xpert® HCV test, authorized by the FDA, allows complete hepatitis C RNA testing at the point-of-care. The test runs on the Xpert® GeneXpert system, enabling any trained healthcare professional, regardless of skill level, to administer the test. This easy-to-use solution provides Lab in a Cartridge™ results when and where they are needed.
The Impact of Point of Care Hepatitis C Testing Globally


The Mobile Kombi Clinic brings HepC Testing to Vulnerable Communities in Australia
“Rapid HCV RNA tests, which detect HCV genetic material, offer the greatest advantage for diagnosis and rapid initiation of treatment as the presence of HCV RNA confirms current infection.6”
Product Resources
Xpert HCV Instructions for Use
Xpert HCV Quick Reference Instructions
Xpert HCV Product Reference Sheet
Xpert US-IVD Test Menu
Connectivity Brochure
Customer Care Brochure
GeneXpert Xpress Brochure
Xpert HCV FAQs
Downloadable Social Media Assets
*IVD. In Vitro Diagnostic Medical Device. May not be available in all countries.
^K2 EDTA Microtainer (BD part number: 365974) not provided in the kit.
1. US Department of Health and Human Services. Viral Hepatitis National Strategic Plan for the United States: a roadmap to elimination for the United States, 2021–2025. Washington, DC: US Department of Health and Human Services; 2020. https://www.hhs.gov/sites/default/files/Viral-Hepatitis-National-Strategic-Plan-2021-2025.pdf
2. Karon C Lewis, Laurie K Barker, Ruth B Jiles, Neil Gupta, Estimated Prevalence and Awareness of Hepatitis C Virus Infection Among US Adults: National Health and Nutrition Examination Survey, January 2017–March 2020, Clinical Infectious Diseases, Volume 77, Issue 10, 15 November 2023, Pages 1413–1415.
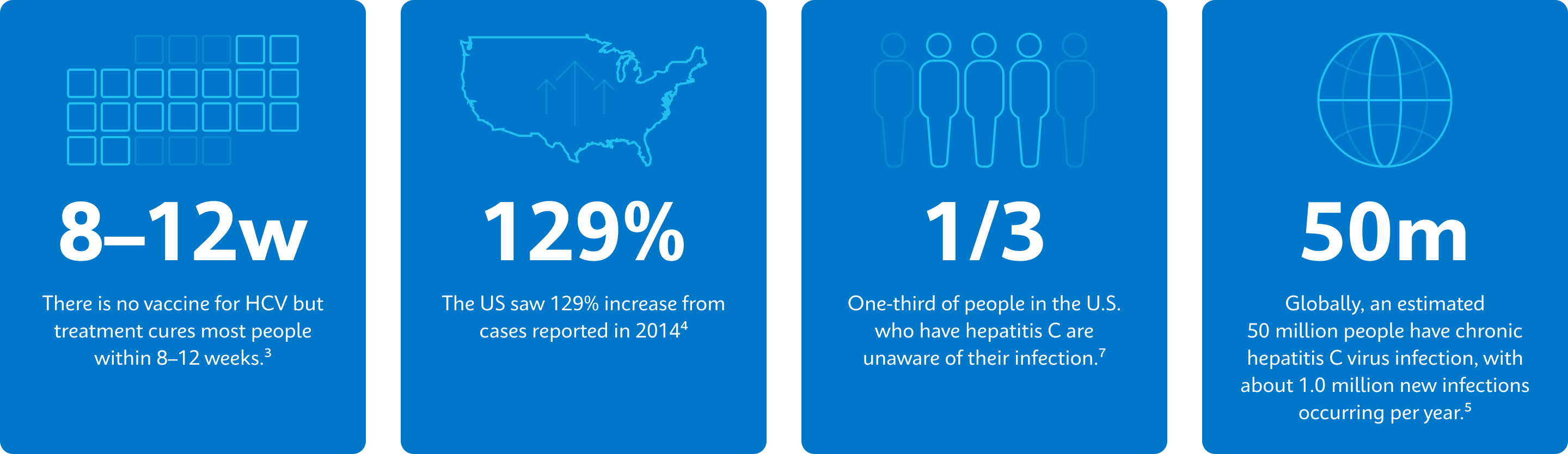
3. Centers for Disease Control (2023, December 23). Treatment of Hepatitis C. CDC https://www.cdc.gov/hepatitis-c/treatment/index.html
4. CDC (2023, August 7). Hepatitis C Surveillance 2021. CDC Viral Hepatitis. Retrieved May 28, 2024, from https://www.cdc.gov/hepatitis/statistics/2021surveillance/hepatitis-c.htm
5. World Health Organization (2024, April 9). Hepatitis C. Retrieved June 4, 2024, from https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
6. Grebely J, Applegate TL, Cunningham P, Feld JJ. Hepatitis C point-of-care diagnostics: in search of a single visit diagnosis. Expert Rev Mol Diagn 2017; 17:1109–15.
7. Centers for Disease Control (2024, March 14). Hepatitis C Surveillance Guidance. CDC Viral Hepatitis. https://www.cdc.gov/hepatitis/statistics/surveillanceguidance/HepatitisC.htm



