Revolution for A Better Way
Cepheid’s Global Access program started in 2011 with the vision for equitable access to revolutionary new molecular diagnostics. Today, Cepheid’s Global Access program reaches every corner of the globe impacted by deadly diseases like Tuberculosis (TB) and HIV and Ebola.

“Xpert MTB/RIF moved the diagnosis of TB and drug resistance from weeks to hours – enabling patients to be identified and treated far more quickly. We have invested heavily in these innovations and will continue to invest in new products critical to eradicating disease.”
David Persing, M.D., Ph.D., EVP and Chief Medical & Scientific Officer

“Xpert MTB/RIF moved the diagnosis of TB and drug resistance from weeks to hours – enabling patients to be identified and treated far more quickly. We have invested heavily in these innovations and will continue to invest in new products critical to eradicating disease.”
David Persing, M.D., Ph.D., EVP and Chief Medical & Scientific Officer
Enpowering Global Health Solutions
Cepheid’s ongoing commitment to invest and innovate continues to improve the standard of care for communities in low- and middle-income countries. Here is how Cepheid makes a difference:
1The Innovation Revolution 2Fostering Partnerships 3Access and Deployment
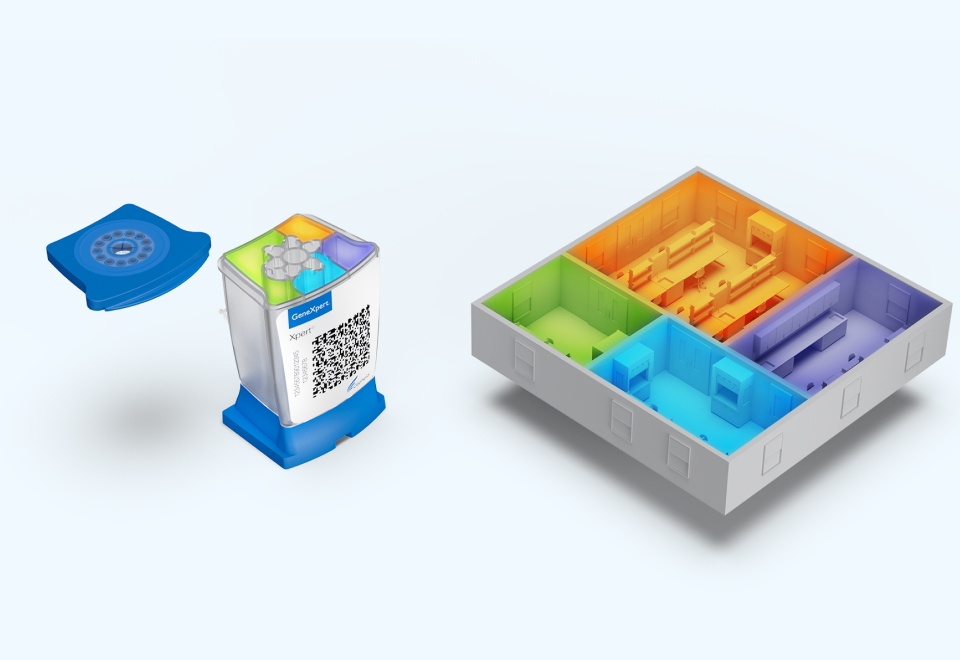
1. The Innovation Revolution
Cepheid’s Lab in a Cartridge™ remains a state-of-the-art molecular diagnostics innovation — replacing multiple large laboratory rooms with small cartridge chambers. Cepheid's groundbreaking Xpert MTB/RIF test was the most significant TB diagnostic innovation in nearly a century, and was quickly endorsed by the WHO1 and heralded by the global community as a game changing diagnostic weapon.
“Investments in GeneXpert platforms have contributed to a massive expansion of cases identified by these rapid diagnostic tests. At a global level, 38% of people newly diagnosed with TB in 2021 were diagnosed with a WHO-recommended diagnostic test, while in 2022, the number rose to 47%. These instruments have been a game-changer.3”
Message from the Executive Director
Global Fund Investments in Health and Laboratory-related Equipment
January 21, 2024
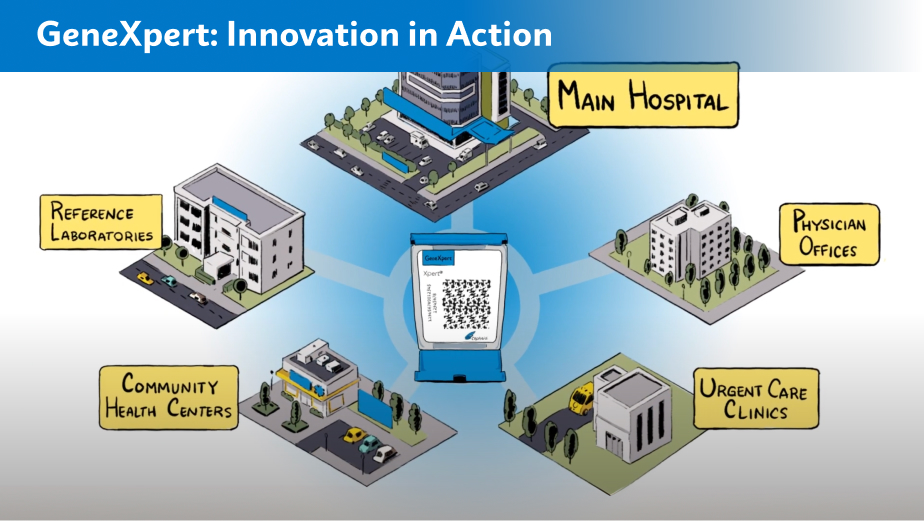
Learn how Cepheid’s innovative approach dramatically impacts patient care.*
* Not all systems shown available worldwide.

Learn how Cepheid’s innovative approach dramatically impacts patient care.*
* Not all systems shown available worldwide.
2. Fostering Partnerships
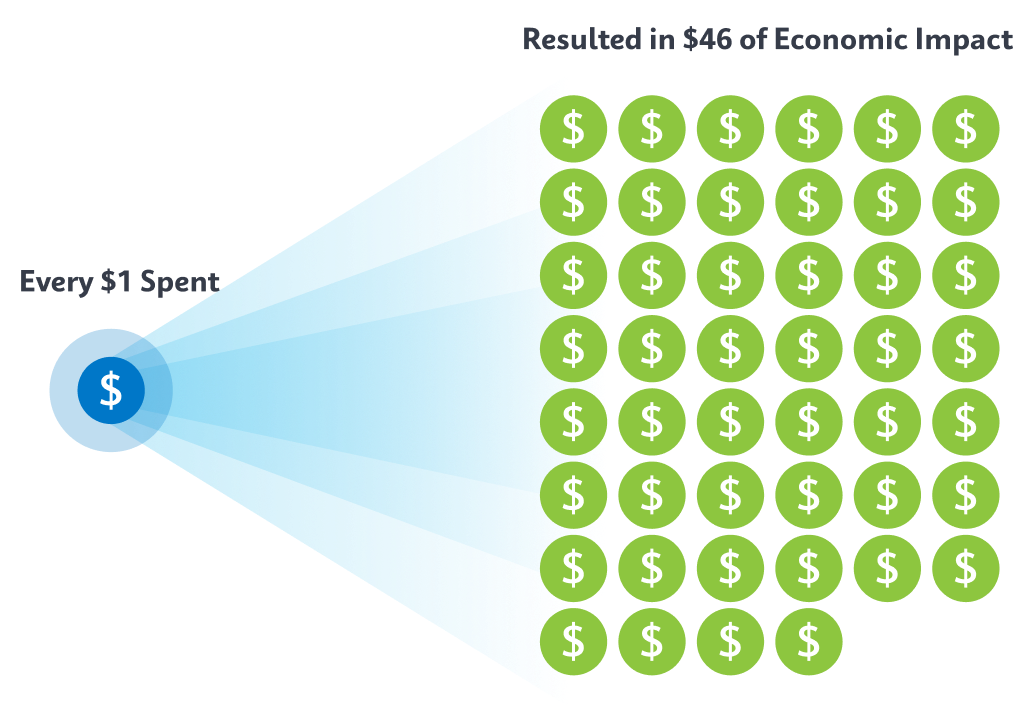
Cepheid’s technology has played a major role in the foundation of economic recovery across low- and middle-income countries. However, it’s the coordinated efforts of donors, policymakers, governments, NGOs, and national and local health systems that are helping change health initiatives to maximize impact. In fact, a recent health economic analysis found that for every $1 of spend on TB programs the result was $46 in economic impact in country.2
“TB is not just a health issue - it is an economic issue, a development issue, and a security issue, and we need strong collaborative efforts from heads of states, private and public sector to really move the needle. We need far more diagnosis, treatment, and prevention. We need more funding, more programs to address barriers to access and more political commitment to help the people who need it most.4”
Peter Sands
Executive Director, Global Fund to Fight AIDS, Tuberculosis and Malaria
TB Innovation Summit
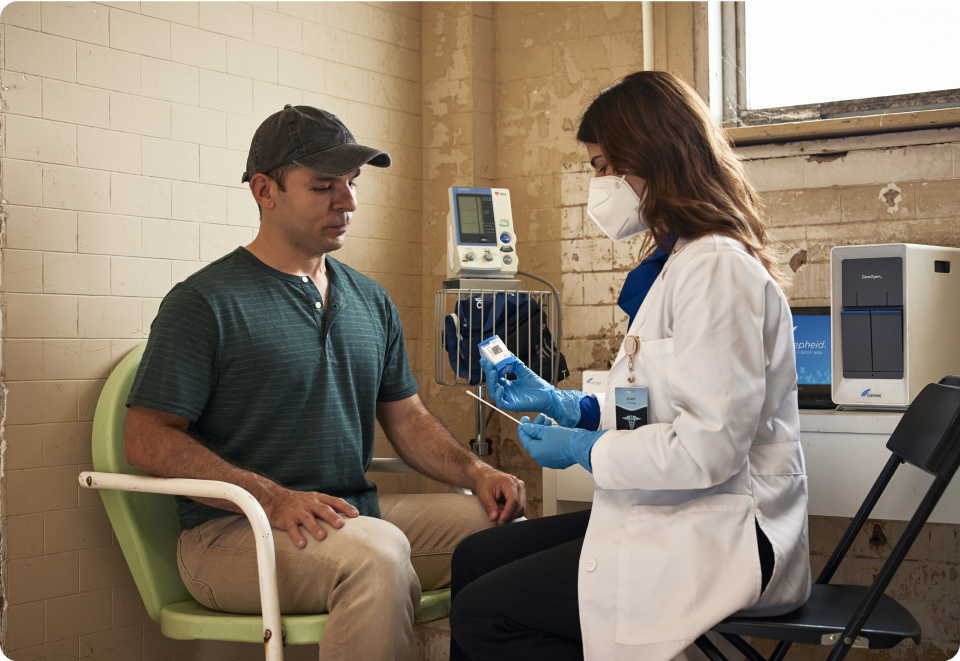
3. Access and Deployment
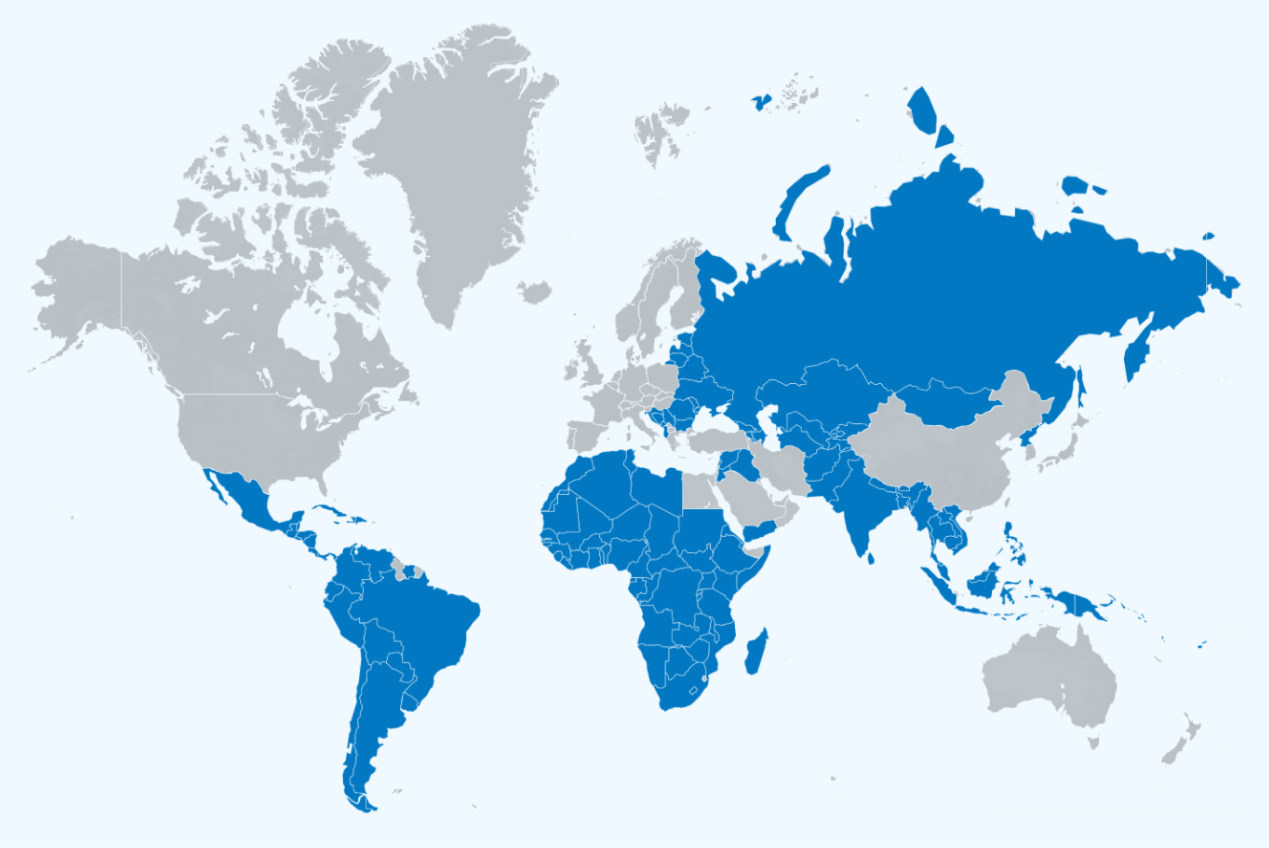
With thousands of GeneXpert systems installed across HBDC, there isn’t a national algorithm in a high-burden country that doesn’t incorporate the GeneXpert as the basis of its TB response. In September of 2023, Danaher (Cepheid's parent company) committed to providing Cepheid's Xpert® MTB/RIF Ultra diagnostic test cartridges for TB at cost, with no profit to Danaher. Universally welcomed across National TB Programs globally, this price reduction further expands access to the technology and the overall impact of the Global Access Program.
Over 140 Countries are eligible for Global Access pricing


"I Want to leave a legacy ... the children of our children will forget about tuberculosis."
See the compelling story of how partnership, collaboration, and intense focus on the elimination of TB are positively impacting the communities of Indonesia.

"I Want to leave a legacy ... the children of our children will forget about tuberculosis."
See the compelling story of how partnership, collaboration, and intense focus on the elimination of TB are positively impacting the communities of Indonesia.
Improving HIV Diagnostics: Stories from the Field

Rapid HIV testing for mothers and babies in rural Tanzania is improving health outcomes

Rapid HIV testing for mothers and babies in rural Tanzania is improving health outcomes
Program History

2006
Cepheid places first GeneXpert® systems
WHO launches Stop TB Strategy on World TB Day

Cepheid, FIND, University of Medicine and Dentistry of New Jersey (Rutgers University), and NIAID collaborate to develop Xpert® MTB/RIF* for TB with a grant from the Bill & Melinda Gates Foundation

2009 - 2010
Xpert MTB/RIF* launches and gets WHO endorsement


2011
Cepheid officially establishes Global Access Program
In response to high rates of TB-HIV co-infection in Global Access countries, Cepheid and FIND partner on development of Xpert HIV-1viral load* (VL) test.

2014
Xpert® HPV* and Xpert HIV-1 VL* tests launch

2015
Cepheid develops and launches Xpert® Ebola test to respond to West African outbreak with funding support from the Paul G. Allen and Bill & Melinda Gates Foundations

Xpert® HIV-1 Qual* and HCV VL* tests launch
2016
WHO prequalifies Xpert HIV-1 Qual*


2017
WHO prequalifies Xpert HCV VL*, Xpert HIV-1 VL*, and Xpert HPV*; Xpert® MTB/RIF Ultra* launches and gets WHO endorsement


2020
Xpert® MTB/XDR* launches to detect multidrug-resistant TB with new 10-color multiplexing technology

Xpert® Xpress SARS-CoV-2^ launches
Global Access Program today
- GeneXpert systems available in multiple modular configuration options
- 15 tests available under Global Access Program, 7 of which are endorsed or pre-qualified by WHO
How We Work
We embed ourselves in the communities we serve to understand their needs, challenges, and preferences and translate these insights into patient-centric solutions: molecular diagnostic systems, tests, and service and support programs. Diseases are not static, so we continuously challenge ourselves to improve existing offerings and to anticipate what the world will need five to ten years from now.
Pushing the envelope for TB diagnostics:
With every launch of a new TB test, Cepheid and its partners have redefined how TB is diagnosed and managed. Since 2006, Cepheid has collaborated with Rutgers University, Foundation for Innovative New Diagnostics (FIND), and National Institute of Allergy and Infectious Disease (NIAID) to harness academic discoveries and scale them for real-world use. The Xpert MTB/RIF* test launched in 2010 with funding support from the Bill & Melinda Gates Foundation. It was a first-of-its-kind molecular test, detecting TB and rifampicin resistance simultaneously. In 2017, Xpert MTB/RIF Ultra* improved on the original test by using detection technology that increased analytical sensitivity by 10-fold and improved detection of rifampicin-related mutations. Xpert MTB/XDR* launched in 2020 using new multiplexing technology to detect resistance to six first- and second-line drugs used for TB treatment.
Instruments and tests alone are not enough, so we strive to provide an ecosystem of awareness and educational initiatives, service, support, and delivery models to strengthen national diagnostic infrastructure.
Accelerating access to Xpert MTB/RIF Ultra:*
Starting in 2017, Cepheid worked with South Africa’s National Health Laboratory System (NHLS) to accelerate access to the new Xpert MTB/RIF Ultra* test. Cepheid’s local Field Application Specialists partnered with the NHLS’ National Priority Programs (NPP) team to make software upgrades required for the test across the instrument network, ensure data transmission into laboratory information systems (LIS) aligned with laboratory requirements, and conduct trainings. The trainings included “train the trainer” sessions for NPP staff, as well as lab employees. In total, Cepheid and NPP employees conducted 70 trainings for more than 200 labs, health centers, and mobile clinics.
We engage directly with donors, policymakers, governments, NGOs, communities, and patients to ensure our work supports global, national, and local health system and disease elimination goals.
Responding to the West African Ebola outbreak:
When the 2014-2016 Ebola outbreak began in West Africa, lack of rapid accurate diagnostics made it difficult to track the outbreak and implement community health measures. Shortly after the World Health Organization (WHO) declared a Public Health Emergency, Cepheid began development of the Xpert Ebola test with a grant from the Paul G. Allen Family Foundation and the Bill & Melinda Gates Foundation. The first Xpert Ebola tests reached health facilities in June 2015, leveraging the existing GeneXpert systems in Sierra Leone, Guinea, and Liberia. Emergency response teams in the field deployed Xpert Ebola for surveillance and diagnosis, establishing detection centers near zones of transmission to prevent further spread.
We speak up for policies and public health initiatives that increase access to molecular testing and reduce health disparities.
Bringing private sector perspective to global disease elimination goals:
Cepheid is a member of the Global Fund and Stop TB Partnership Private Sector Constituencies. In these forums, Cepheid advocates for inclusion of high-quality diagnostics and patient-centered care in global disease elimination plans. The Stop TB Partnership Private Sector Constituency has released white papers on topics including connectivity, sustainable investment, and resilient health systems.
To request information on our AccessCare Program please complete the following form:
Contact Us
- For media inquiries, contact media.communications@cepheid.com.
- For product, partnership, or general inquiries contact ordersinternational@cepheid.com
Resources
For more information on the Global Access Program and our offerings, please refer to the following:
* CE-IVD. In Vitro Diagnostic Medical Device. May not be available in all countries. Not available in the United States.
^ For use under an Emergency Use Authorization in the United States
IVD. In Vitro Diagnostic Medical Device. May not be available in all countries
1. https://www.who.int/publications/i/item/9789241506335
2. Journal of Benefit-Cost Analysis (2023) https://www.cambridge.org/core/journals/journal-of-benefit-cost-analysis/article/one-million-lives-saved-per-year-a-costbenefit-analysis-of-the-global-plan-to-end-tuberculosis-20232030-and-beyond/A74F0D10F1017092A250EB604ED39B1B
3. Message from the Executive Director: Global Fund Investments in Health and Laboratory-related Equipment; January 21, 2024 https://www.theglobalfund.org/en/oig/updates/2024-01-26-message-executive-director-global-fund-investments-health-laboratory-related-equipment/
4. Peter Sands, Executive Director, Global Fund to Fight AIDS, Tuberculosis and Malaria.
From the TB Innovation Summit https://www.stoptb.org/news/global-health-and-business-leaders-pledge-major-commitments-to-end-tuberculosis