Confidence. It comes from knowing that you have the right partner for IVDR compliance.
Test portfolio on track to be converted to IVDR
What is In Vitro Diagnostic Regulation (IVDR)?
In Vitro Diagnostic Regulation (IVDR – 2017/746) is the current regulatory basis for placing on the market, making available, and putting into service in vitro diagnostic medical devices in the European market.1 IVDR replaces the previous regulation, the In Vitro Diagnostic Directive (IVDD – 98/79/EC).
IVDR – 2017/746 was published in the Official Journal of the EU on 5 May 2017 and entered into force on 26 May 2017. As a European regulation, it is currently effective in all EU member states and EEA states.
The new regulations aim to ensure the effectiveness and safety of medical device marketing within the EU. Cepheid is diligently working to ensure all relevant products are covered under the new ordinance.

What is covered under IVDR?
Any in vitro diagnostic medical device considered a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software, or accessory, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally to provide information on one or more of the following:
- Concerning a physiological or pathological process or state
- Concerning congenital physical or mental impairments
- Concerning the predisposition to a medical condition or a disease
- To determine the safety and compatibility with potential recipients
- To predict treatment response or reactions
- To define or monitor therapeutic measures
- Specimen receptacles shall also be deemed in vitro diagnostic medical devices
Objectives of IVDR
- To ensure patient safety
- To ensure seamless functioning of the internal market
- To provide a regulatory framework that supports innovation and competitiveness of the European medical device industry
- To provide harmonization of regulations across all member states of the EU
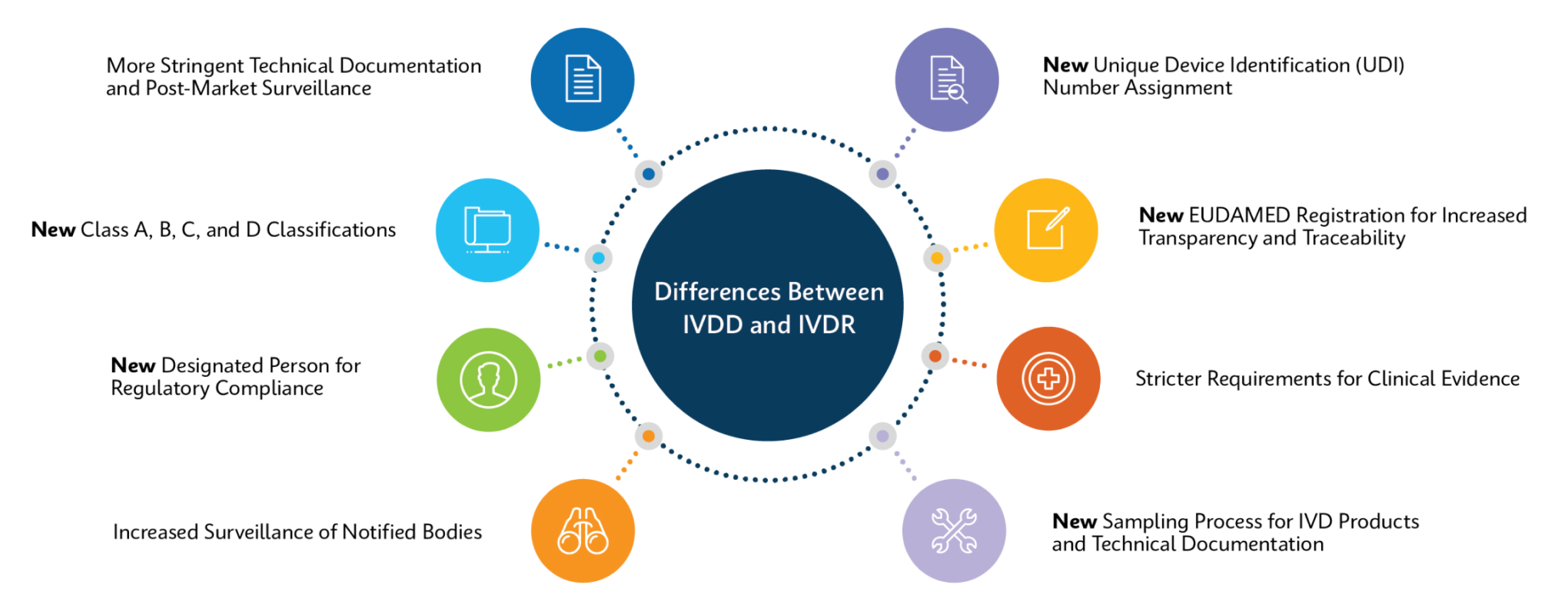
How Does IVDR Impact Customers?
Cepheid is working to ensure all relevant products are IVDR-compliant and have processes in place for recertification to ensure product continuity for all our customers.
For most products, changes will be minimal: same product code, same performance, and validation requirements will be kept to a minimum. For a few products, there will be a change in catalog number, which may entail more extensive update/validation processes.
Changes may include but not limited to:
- For devices that require notified body oversight under IVDR, IFU changes and Xpert test kits will include the CE Notified Body Number alongside the CE mark
- Intended purpose statements
- Warnings or contraindications
- Additions or alterations to sample types, stability, interfering substances, linearity, reference ranges, specificity, and sensitivity
- Updates to supporting references
- Changes to product labeling
Before a product is launched, all users will receive a letter detailing these changes and Cepheid's teams will be mobilized to help make this changeover as smooth as possible.

Cepheid shares the values of transparency and health protection for patients and users championed by the IVDR and is committed to supporting all our customers and distributors during this transition phase.
Cepheid’s Key Milestones Progression
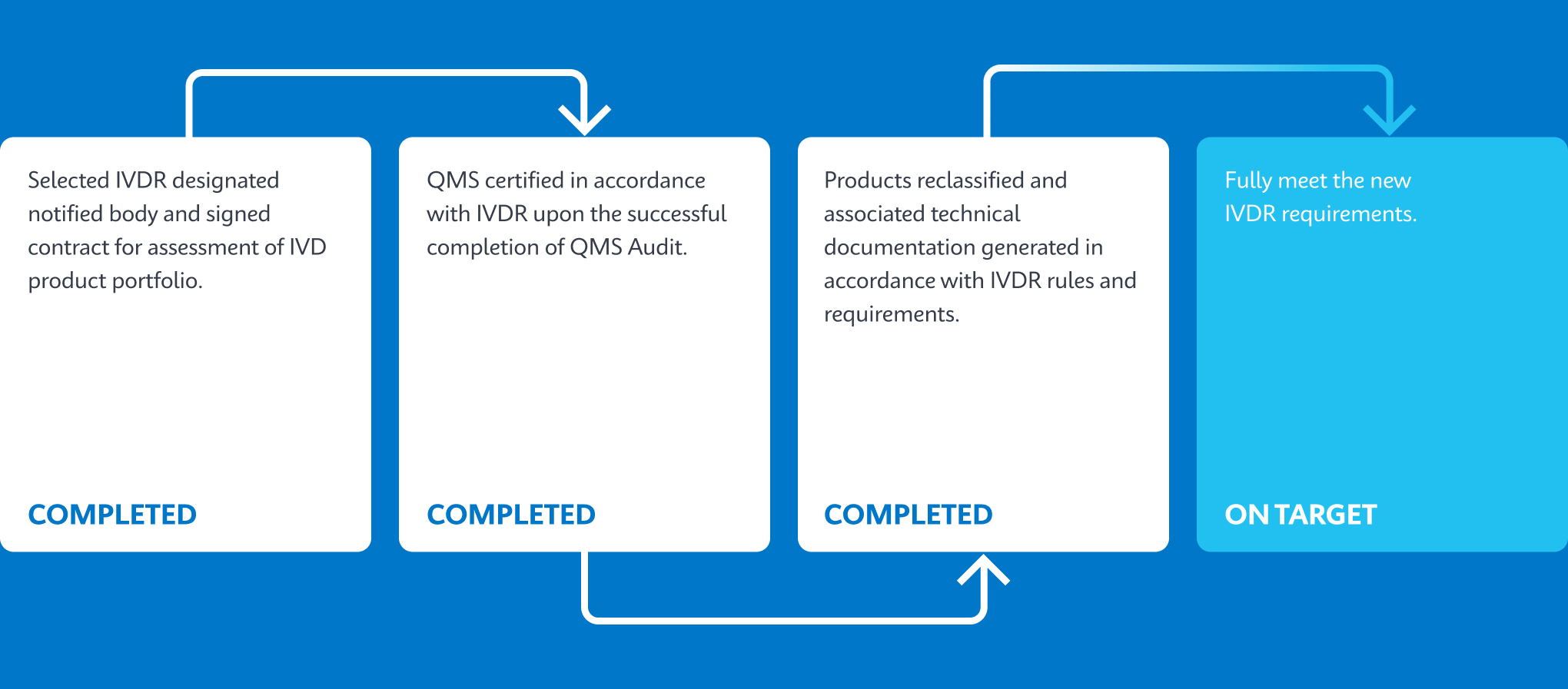
Test Portfolio on Track To Be Converted To IVDR
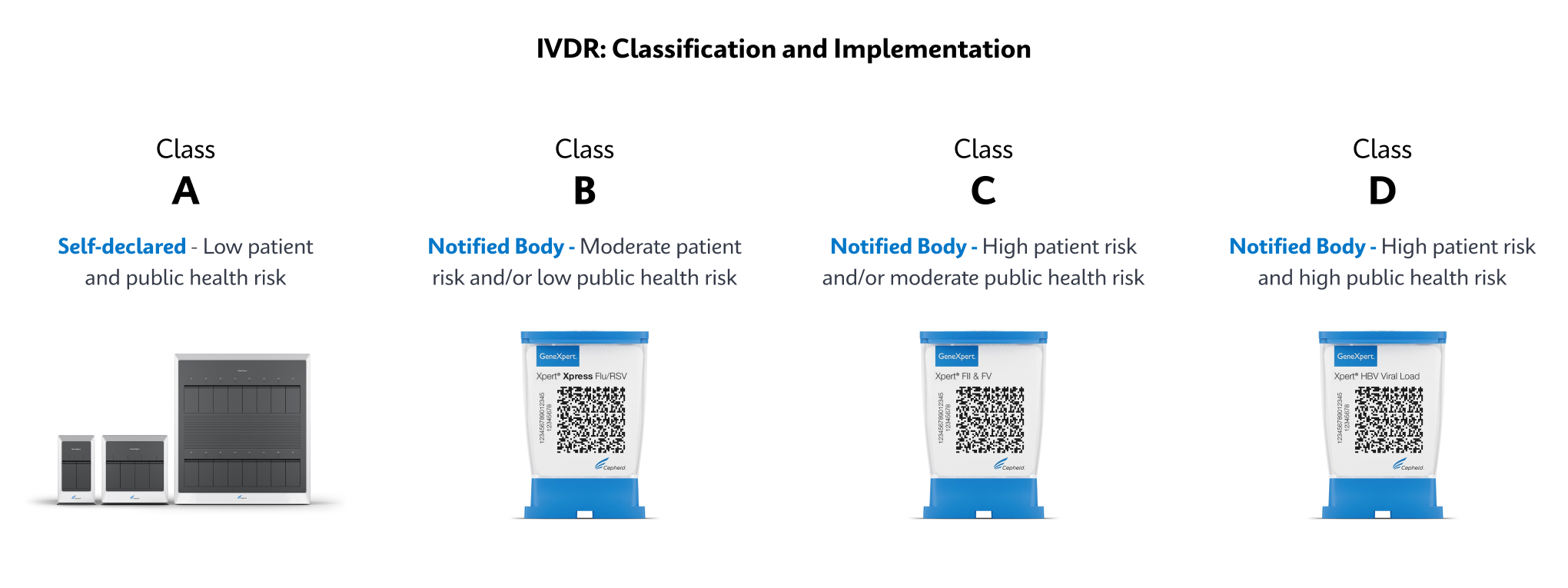

IVD. In Vitro Diagnostic Medical Device. May not be available in all countries
1. In vitro Diagnostic Medical Device Regulation (IVDR). (2021). TUV. https://www.tuvsud.com/en-us/industries/healthcare-and-medical-devices/medical-devices-and-ivd/medical-device-market-approval-and-certification/eu-in-vitro-diagnostic-medical-device-regulation/faqs-in-vitro-diagnostic-medical-device-regulation-ivdr
2. Regulation (Eu) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU
3. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32017R0746&from=EN