1
* Not all systems shown available worldwide.
2

Budi Gunadi Sadikin
Minister of Health
Republic of Indonesia
3
Program History

2006
Cepheid places first GeneXpert® systems
2006
WHO launches Stop TB Strategy on World TB Day

2006
Cepheid, FIND, University of Medicine and Dentistry of New Jersey (Rutgers University), and NIAID collaborate to develop Xpert® MTB/RIF* for TB with a grant from the Bill & Melinda Gates Foundation

2009–2010
Xpert MTB/RIF* launches and gets WHO endorsement
Global Access
2011
Cepheid officially establishes Global Access Program

2006
In response to high rates of TB-HIV co-infection in Global Access countries, Cepheid and FIND partner on development of Xpert HIV-1viral load* (VL) test.


2014
Xpert® HPV* and Xpert HIV-1 VL* tests launch

2015
Cepheid develops and launches Xpert® Ebola test to respond to West African outbreak with funding support from the Paul G. Allen and Bill & Melinda Gates Foundations
2016
WHO prequalifies Xpert HIV-1 Qual*

2017
WHO prequalifies Xpert HCV VL*, Xpert HIV-1 VL*, and Xpert HPV*; Xpert® MTB/RIF Ultra* launches and gets WHO endorsement

2020
Xpert® MTB/XDR* launches to detect multidrug-resistant TB with new 10-color multiplexing technology

2020
Xpert® Xpress SARS-CoV-2" launches

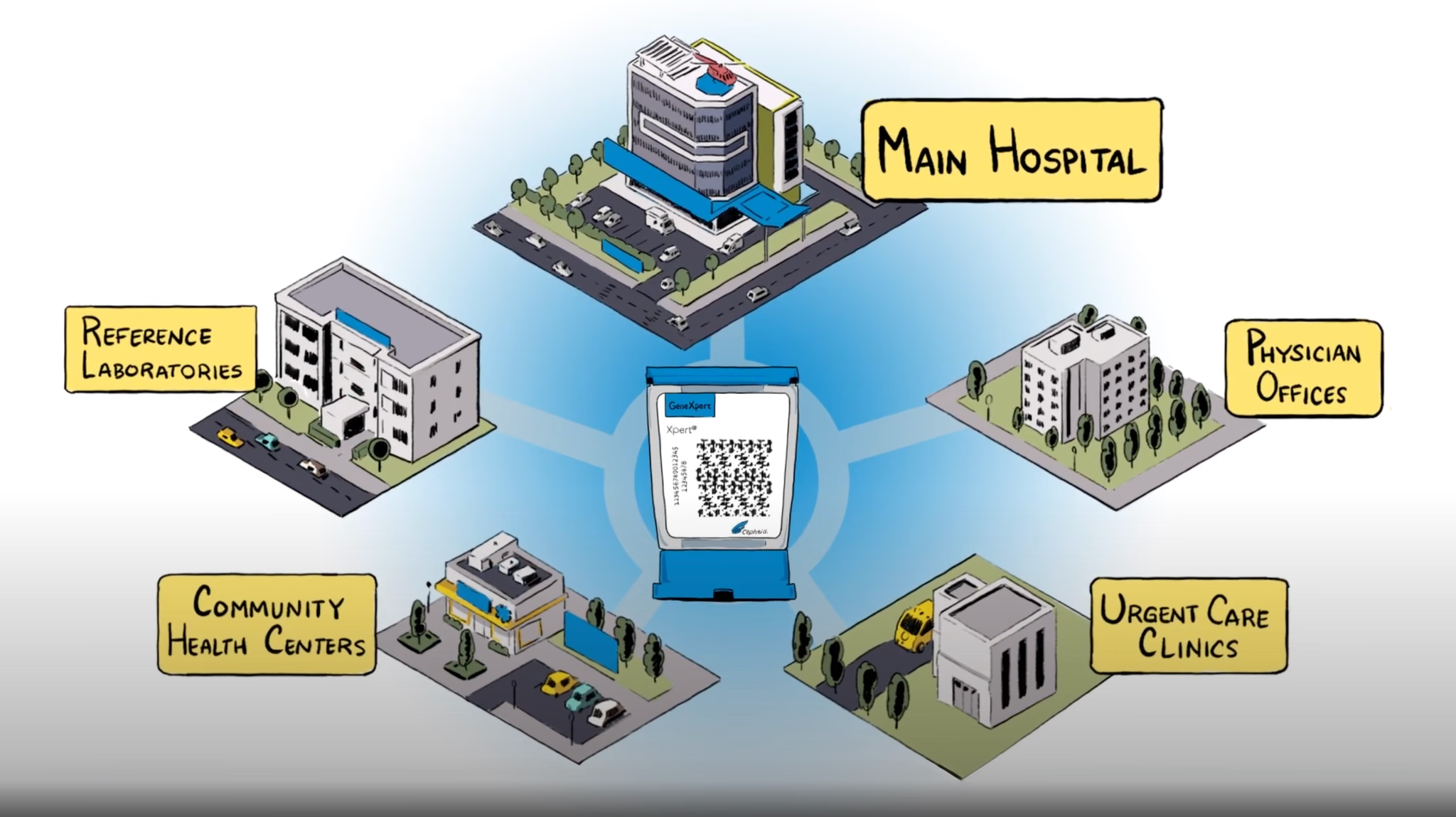
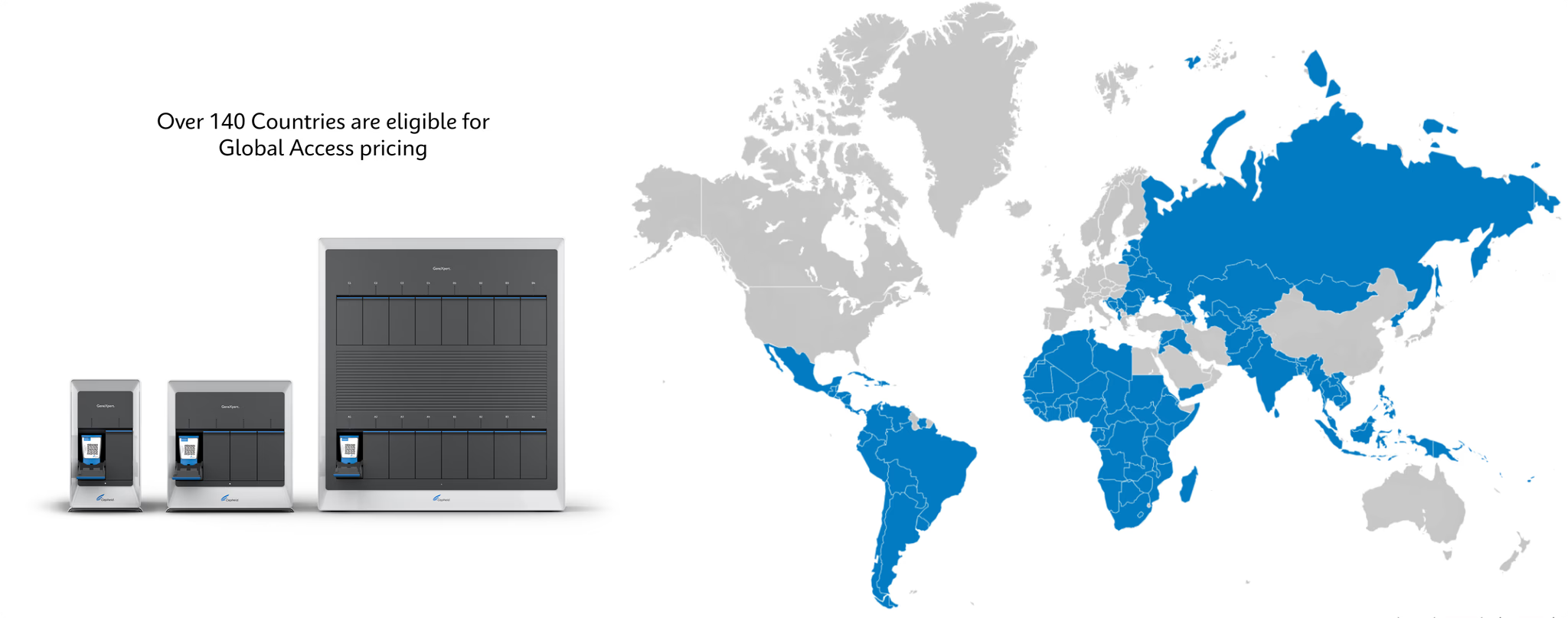
Global Access Program Today
GeneXpert systems available in multiple modular configuration options 15 tests available under Global Access Program, 7 of which are endorsed or pre-qualified by WHO
* CE-IVD. In Vitro Diagnostic Medical Device. May not be available in all countries. Not available in the United States.
IVD. In Vitro Diagnostic Medical Device. May not be available in all countries
^ For use under an Emergency Use Authorization in the United States
Dr. Tereza Kasaeva, Director, Global Tuberculosis Programme, WHO World TB Day 2023, Interview with Dr. Tereza Kasaeva
https://www.youtube.com/watch?v=AL_t8LZkD8Y
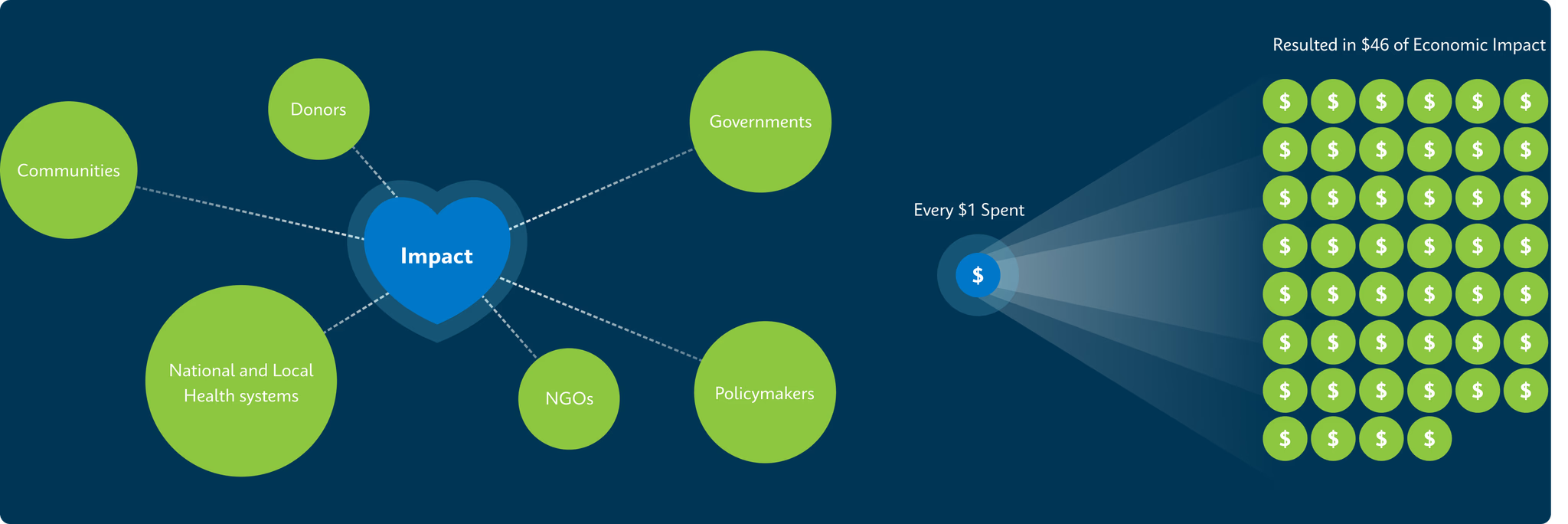
2. Journal of Benefit-Cost Analysis (2023) https://www.cambridge.org/core/journals/journal-of-benefit-cost-analysis/article/one-million-lives-saved-per-year-a-costbenefit-analysis-of-the-global-plan-to-end-tuberculosis-20232030-and-beyond/A74F0D10F1017092A250EB604ED39B1B
3. Message from the Executive Director: Global Fund Investments in Health and Laboratory-related Equipment; January 21, 2024
https://www.theglobalfund.org/en/oig/updates/2024-01-26-message-executive-director-global-fund-investments-health-laboratory-related-equipment/
4. Peter Sands, Executive Director, Global Fund to Fight AIDS, Tuberculosis and Malaria.
From the TB Innovation Summit https://www.stoptb.org/news/global-health-and-business-leaders-pledge-major-commitments-to-end-tuberculosis