8m Read
May 28, 2025
COMMUNITY AND GLOBAL HEALTH
Article
Integration of POC Viral Diagnostics with Substance Use Disorder Treatment to Improve Hepatitis C Infection Outcomes
The Syndemic of Hepatitis C Infection and Opioid Use Disorder
In the United States, an estimated 2.4 – 3 million people have hepatitis C virus (HCV) infection, and the most commonly reported risk factor for HCV infection is injection drug use.1
HCV rates in the U.S. have increased concurrently with the increase in injection opioid use, with most new infections occurring in young people who inject drugs. The majority of HCV cases are not diagnosed and not reported due to a lack of access to appropriate testing.
Because of the high risk of acquisition of HCV infection in people who inject drugs, it makes sense to co-localize access to HCV testing and treatment with substance use disorder treatment and services.
Patients accessing these services do not always have reliable transportation in a rural community, and multiple visits to a medical facility to get tested, diagnosed, and initiated treatment for HCV are extremely burdensome.
In the KeYTreat study by Dr. Jennifer Havens of the University of Kentucky College of Medicine, HCV testing and treatment were offered at a site that offers syringe services for people who inject drugs.
“The ability to access treatment is currently hampered for the more marginalized groups in a very small community that was very well characterized by some of the research we had done showing that there were high rates of hepatitis transmission among people who inject drugs, and mainly who were injecting prescription opioids, at least initially,” indicated Havens. “If we are going to eliminate hepatitis C, this needs to be the group that is targeted for treatment.”
In this study, co-locating HCV testing and treatment with a syringe service program significantly increased diagnosis and treatment rates.
Despite this, according to the National Substance Use and Mental Health Services Survey, only a third of the 15,000 substance use disorder treatment facilities in the U.S. offer HCV testing or testing for HIV or HBV.2
HCV Diagnostic Challenges
Getting patients tested, diagnosed, and linked to care and treatment for HCV infection is a challenge that needs to be overcome to reach the National Hepatitis C Elimination goals.
In 2023, Drs. Rachael Fleurence and Francis Collins, then part of the Biden White House team, outlined three priorities for a National Hepatitis C Elimination Plan:
1. Accelerating the availability of point-of-care HCV RNA diagnostic tests, allowing for single-visit test and treatment programs in decentralized settings such as substance use disorder clinics and mobile outreach vans
2. Provide broad access to HCV treatments
3. Build a comprehensive public health effort that includes clinical and community partners to engage and ultimately cure people of HCV infection.
Testing is a critical step in this effort. HCV diagnostic testing has been performed via a two-step process that includes a “screening” test for HCV antibodies, performed at the point of care or in a centralized laboratory. A positive HCV antibody result indicates that the patient has had an HCV infection but does not differentiate between a past, resolved infection and a current infection.
If the antibody test is positive, RNA-based testing must be performed to confirm active infection; until recently, this testing has only been available in centralized laboratories. The testing algorithm can take days to weeks to complete, requires venipuncture, and requires multiple healthcare visits for the patient to receive their diagnosis and begin treatment. In addition, because testing starts with an antibody-based test, patients who are early in infection and have not yet developed antibodies but can transmit the infection will be missed
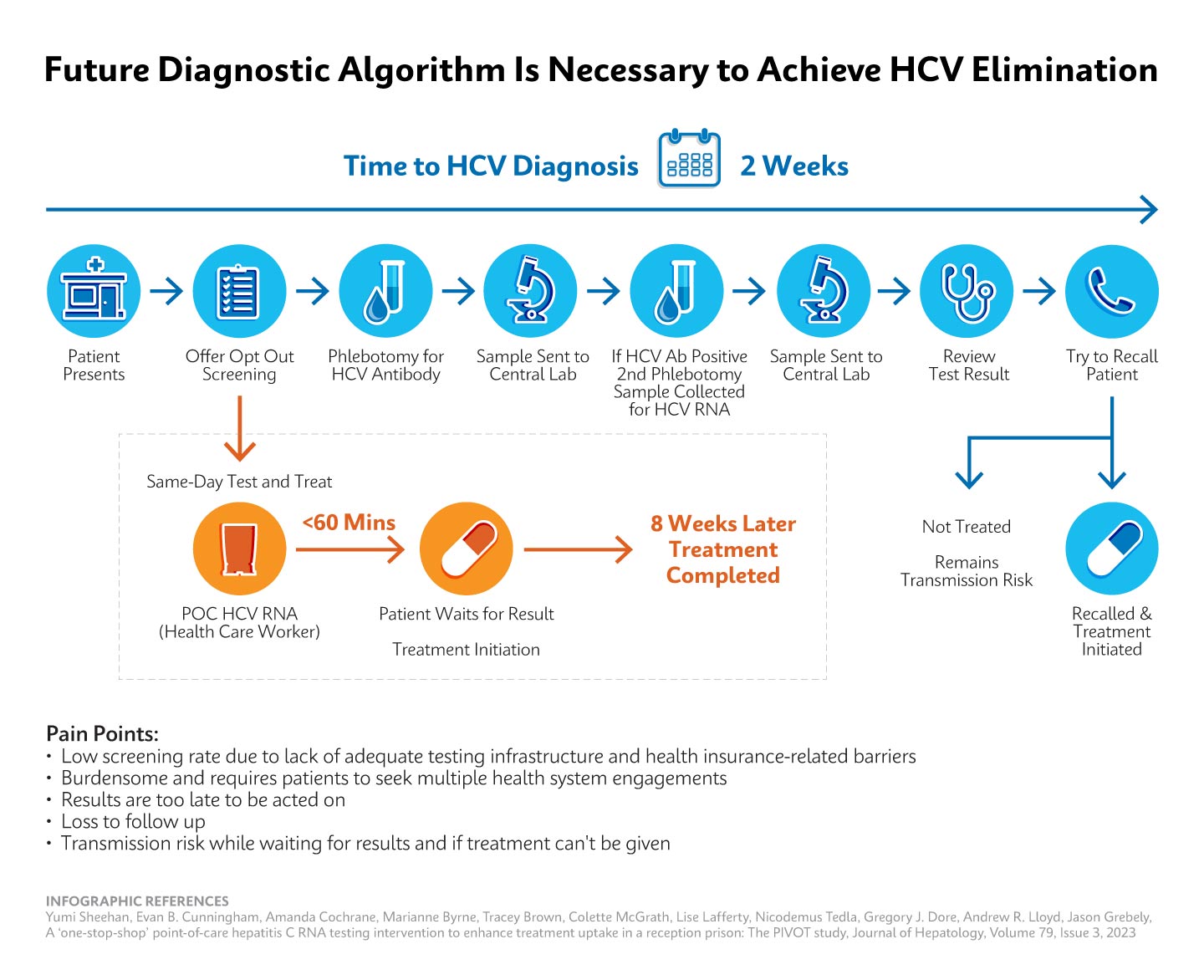
This requires the patient to engage in multiple visits over time and patients are lost at each step in the clearance cascade.
Using data from commercial laboratories from 2013-2022, the CDC analyzed the HCV clearance cascade and found that, at best, 34% of people diagnosed with HCV infection reached viral clearance. At each step of the test to treat to cure process, patients are lost
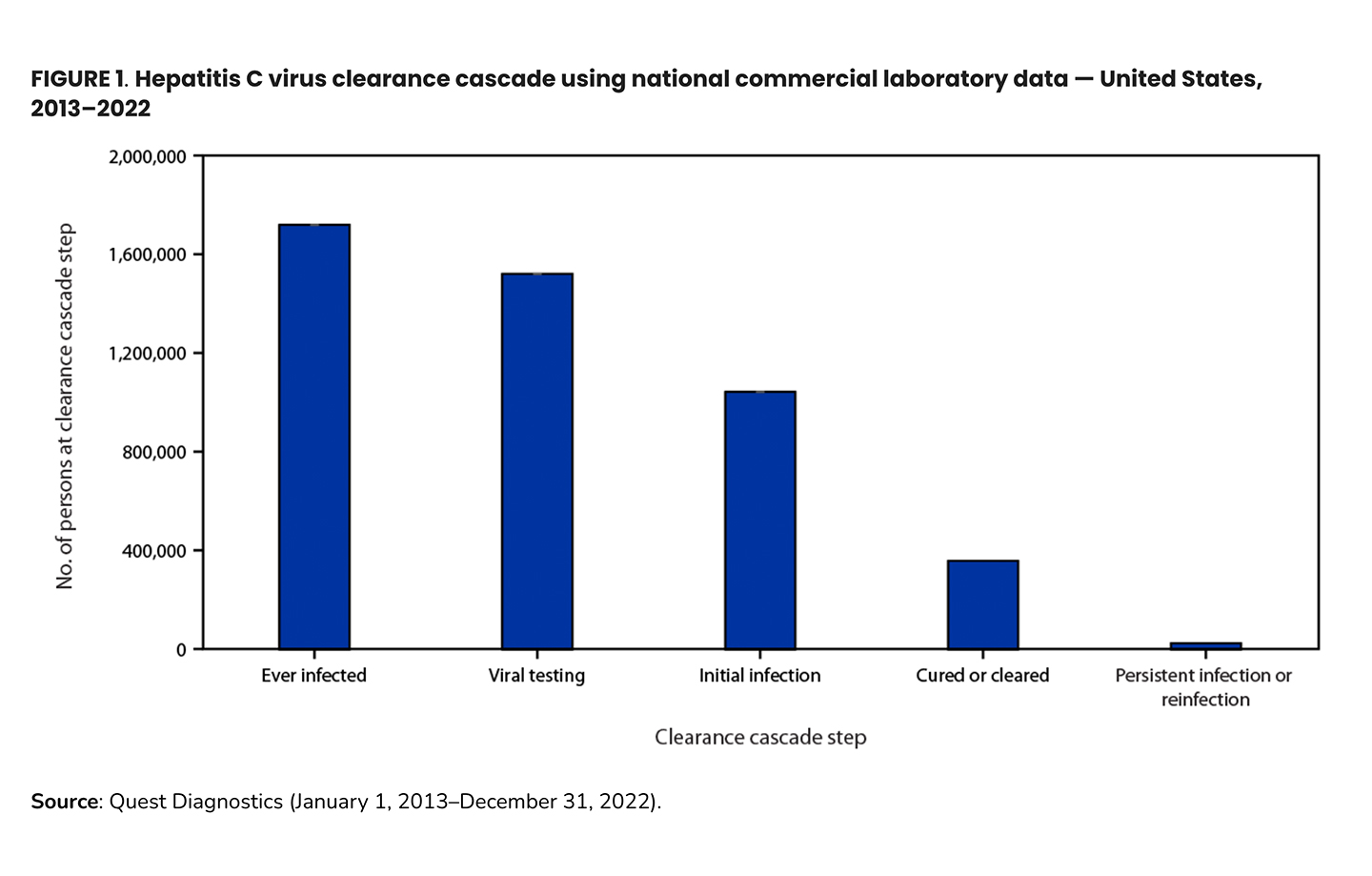
In this analysis, 88% of people with evidence of HCV infection (i.e. positive HCV antibody test) were appropriately tested for current infection with an RNA test (in this dataset, antibody and RNA testing were performed at the same commercial laboratory). Of those with a positive RNA result (current HCV infection), only one-third had evidence of cure. In younger adults (20-39 years), this was less than one in four patients, and the viral clearance prevalence for those in this age group with Medicaid or unspecified coverage is one in six. This demonstrates a gap in access to diagnostic (RNA) testing and treatment in younger adults.3
The two-step testing strategy leaves a fair amount of burden on the patient. For patients with concurrent substance use disorder, this can be overwhelming, and many patients who receive antibody screening testing are lost to follow-up before they can be tested for HCV RNA and diagnosed. It is estimated that one in three people with HCV are unaware of their infection.4
This has consequences for the patient who is not treated for a deadly but curable disease, but also for public health, as patients with untreated HCV infections (the majority of HCV infections are asymptomatic) can unknowingly transmit the disease to others.
Importance of Accurate Testing with Same-day Results in Appropriate Settings
Eliminating HCV infection in the United States is achievable but requires drastic changes in the availability of test and treatment programs. Diagnostic testing in low threshold settings is important, and results should be available to the patient on the same day so that patients with active infection can be immediately linked to care and treatment.
In their Dear Colleague letter,2 Miriam Delphin-Rittmon, Ph.D., then Assistant Secretary for Mental Health and Substance Use and leader of the Substance Abuse and Mental Health Services Administration (SAHMSA), and Mandy Cohen, M.D., M.P.H., then Director, Centers for Disease Control and Prevention (CDC), discuss the connection between injection drug use and acquisition of hepatitis C, hepatitis B, and HIV, the prevalence of these diseases among people who inject drugs, and CDC’s recommendations for HIV, HCV, and HBV testing for people who inject drugs.
CDC recommends routine periodic testing for HCV in patients with ongoing risk factors for infection, and this testing can start with an RNA test (“viral first testing”) in patients with suspicion for exposure to HCV within the prior six-month period. Risk factors include injecting drugs and sharing equipment.
Drs. Delphin-Rittmon and Cohen encourage substance use disorder treatment facilities to integrate testing for HCV, HIV, and HBV into the services they offer clients. They highlight recent advances in point-of-care diagnostics that support such integration in these decentralized settings, including Cepheid’s recently FDA-cleared fingerstick POC HCV RNA test, Xpert HCV, that enables same-day test and treat/test and link to care programs.
A Cepheid Solution
In June, 2024, the first diagnostic test for HCV infection for use in the point-of-care (CLIA-waived) setting received FDA approval. The Cepheid Xpert® HCV test does not require venipuncture; it is performed using blood obtained from a simple fingerstick procedure and can be done outside traditional clinical settings by non-medical personnel. The test takes 41-60 minutes to run and performs comparably to tests performed in centralized laboratories.
This breakthrough was made possible by collaboration and public-private partnerships that included the NIH, the Independent Test Assessment Program, the White House, CDC, FDA, CMS, and many other partners within and outside the government.6
The Xpert HCV test is a diagnostic test that detects HCV RNA and can be used in decentralized settings, such as in substance use disorder clinics, syringe service programs, and in other settings that represent a low-threshold environment for patients with concurrent SUD to present for testing and care.
With the availability of a point-of-care diagnostic and well-tolerated oral treatment, the tools to reach HCV elimination are ready.
Conclusion and Call to Action
Same-day test and treat models of care are now possible in the U.S. With the recent FDA authorization of Xpert HCV, we can now diagnose HCV with a single test that can be performed at the POC, and patients who are diagnosed with HCV can leave their visit with treatment in hand. The tools to implement this test-and-treat model with a public health framework to support patients, providers, and communities is needed. Funding and mechanisms to pay for testing and drug treatment is necessary. Support to enable test and treat models in settings that have not done this work before is critical, and mechanisms to collect and report public health data in these settings must be developed and implemented.
Innovative solutions and collaborations between healthcare organizations, government bodies, and regulatory agencies pave the way for improved access and better diagnostic solutions. By addressing the challenges and implementing evidence-based strategies, we can work towards a future where HCV elimination becomes a reality.
References:
- SAMHSA and CDC Dear Colleague Letter. Accessed April 1, 2025. https://www.cdc.gov/hepatitis/media/pdfs/2024/12/SAMHSA-CDC-DCL-HIV-Viral-Hepatitis-Testing-December-2024-508c_FINAL.pdf
- Substance Abuse and Mental Health Services Administration. (2023). National Substance Use and Mental Health Services Survey (N-SUMHSS) 2022: Annual Detailed Tables (SAMHSA Publication No. PEP23-07-00-002). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data/.
- Wester C, Osinubi A, Kaufman HW, et al. Hepatitis C Virus Clearance Cascade — United States, 2013–2022. MMWR Morb Mortal Wkly Rep 2023;72:716–720. DOI: http://dx.doi.org/10.15585/mmwr.mm7226a3
- Lewis KC, Barker LK, Jiles RB, Gupta N. Estimated Prevalence and Awareness of Hepatitis C Virus Infection Among US Adults: National Health and Nutrition Examination Survey, January 2017-March 2020. Clin Infect Dis. 2023 Nov 17;77(10):1413-1415. doi: 10.1093/cid/ciad411. Erratum in: Clin Infect Dis. 2024 Mar 20;78(3):807-808. doi: 10.1093/cid/ciad783. PMID: 37417196; PMCID: PMC11000503.
- Wester C, Osinubi A, Kaufman HW, et al. Hepatitis C Virus Clearance Cascade — United States, 2013–2022. MMWR Morb Mortal Wkly Rep 2023;72:716–720. DOI: http://dx.doi.org/10.15585/mmwr.mm7226a3.
- A Breakthrough in HCV Elimination. Coalition for Global Hepatitis Elimination. Accessed April 4, 2025. https://www.globalhep.org/sites/default/files/content/webinar/files/2024-12/Oct%20NIH%20CDC%20Webinar%20POC%20PCR%20Synthesis%20Report.pdf
Read Next
MORE







